The evaluation of a digital clinical solution is long and difficult because it requires expertise in various fields including clinical study methodology, embedded technology, clinical relevance, privacy policy, ergonomics and more. This lack of expertise still leads all too often to not only the support of immature solutions, but as well the neglecting of quality solutions.
We designed the MDS score (Medical Digital Score Solution) with the help of 130 French publishers of e-health solutions, health establishments, doctors, health authorities’ recommendations and patient associations. We then evaluated the MDS score as part of an experiment and published the results in July 2022 in JMIR (Journal of Medical Internet Research): https://pubmed.ncbi.nlm.nih.gov/35788102/ – « Assessing a New Prescreening Score for the Simplified Evaluation of the Clinical Quality and Relevance of eHealth Apps : Instrument Validation Study » – Wagneur et al.
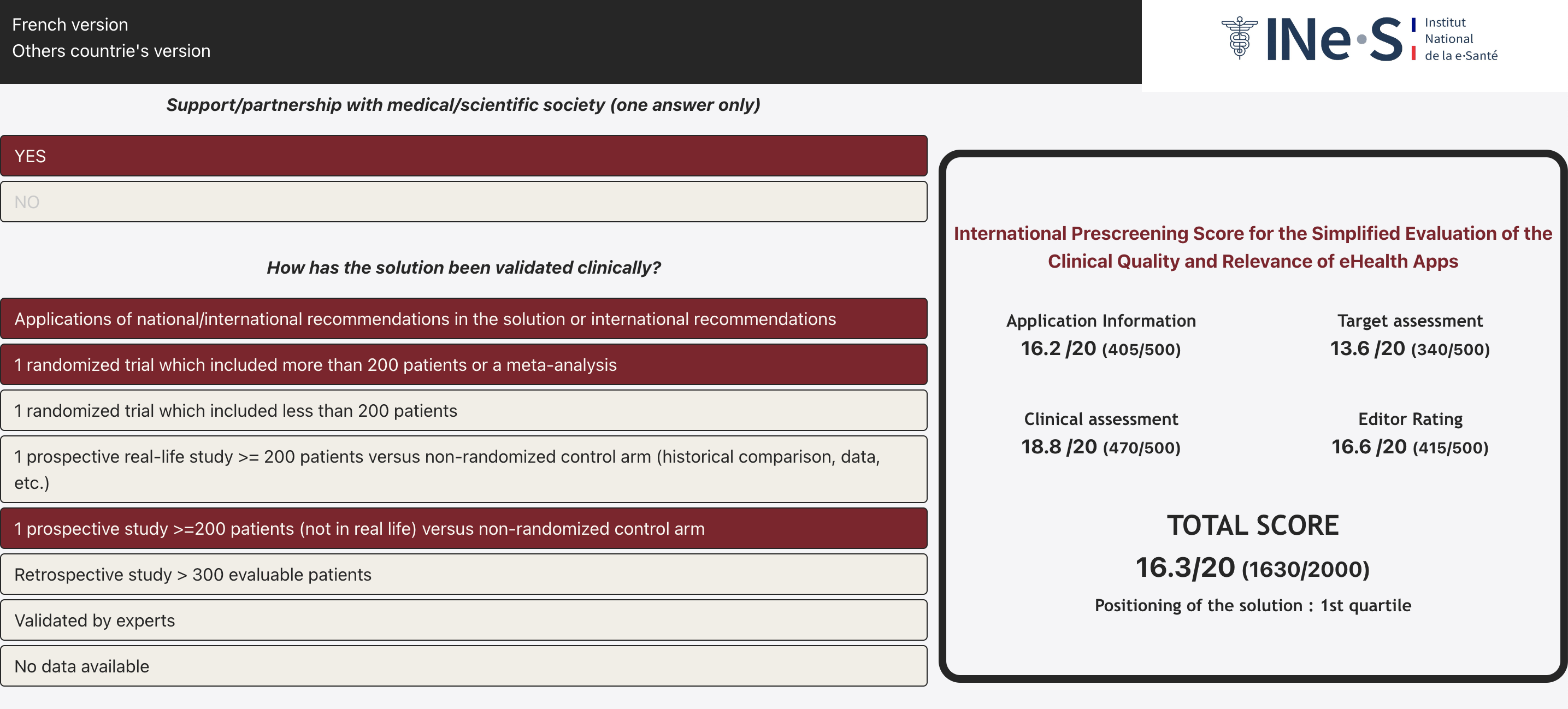
MDS is a prescreening score for the simplified evaluation of the clinical quality and relevance of eHealth Apps for the French market that allows the user to evaluate various criteria, through 26 questions. The evaluation categories include:
- the medical specialty and claims of the clinical application (excluding well-being) (score up to 500 points)
- the target users of the solution (score up to 500 points)
- the clinical evaluation of the solution (score up to 500 points)
- the editor (software publisher) (score up to 500 points)
- the likelihood of obtaining reimbursement
For a total score of up to 2000 points
The MDS score thus allows to:
– guide solution designers toward the best R&D choices
– help in the selection and comparison of solutions with relevant and scalable reproducible criteria
– help healthcare establishments, prescribers, and institutional structures quickly assess solutions before deploying them
– compare solutions with common evaluation criteria
– enable INeS (National e-Health Institute in France) experts recommend solutions for digital health awards.
Since the scoring tool was developed for France and assessments in other countries will differ in terms of legislation, reimbursement conditions and market access, we created a multi-country version of the score, which allows evaluation of solutions outside of France by adapting question’s weightings to those applied internationally.
There are now 2 versions of the MDS scoring tool.
- The French version considers only French national legislation and specificities.
- The international version enables evaluation in other countries with less specific evaluation criteria than in France
*The most relevant countries eligible to MDS scoring assessment are: Canada, Europe Union except France, United Kingdom, USA